Normality (chemistry)
In chemistry, the normality of a solution is defined as the molar concentration 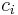 divided by an equivalence factor
divided by an equivalence factor 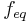 :
:
- normality
Contents |
Units
The unit symbol "N" is used to denote "mol/L" when referring to normality. Alternatively, the symbol "Eq/L" is sometimes used. Although losing favor, medical reporting of serum concentrations in "mEq/L" (=0.001 N) still occurs.
Usage
There are three common areas where normality is used as a measure of reactive species in solution:
- In acid-base chemistry, normality is used to express the concentration of protons (H+) or hydroxide ions (OH-) in a solution. Here,
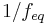 is an integer value. Each solute can produce one or more equivalents of reactive species when dissolved.
is an integer value. Each solute can produce one or more equivalents of reactive species when dissolved. - In redox reactions, the equivalence factor describes the number of electrons that an oxidizing or reducing agent can accept or donate. Here,
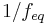 can have a fractional (non-integer) value.
can have a fractional (non-integer) value. - In precipitation reactions, the equivalence factor measures the number of ions which will precipitate in a given reaction. Here,
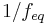 is an integer value.
is an integer value.
Examples
Normality can be used for acid-base titrations. For example, sulfuric acid (H2SO4) is a diprotic acid. Since only 0.5 mol of H2SO4 are needed to produce 1 mol of H+, the equivalence factor is:
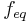 (H2SO4) = 0.5
(H2SO4) = 0.5
If the concentration of a sulfuric acid solution is c(H2SO4) = 1 mol/L, then its normality is 2 N. It can also be called a "2 normal" solution.
Similarly, for a solution with c(H3PO4) = 1 mol/L, the normality is 3 N because phosphoric acid contains 3 acidic H atoms.
Criticism
Normality is an ambiguous measure of the concentration of a solution. It needs a definition of the equivalence factor, which depends on the definition of equivalents. The same solution can possess different normalities for different reactions. The definition of the equivalence factor varies depending on the type of chemical reaction that is discussed: It may refer to equations, bases, redox species, precipitating ions, or isotopes. For example, a solution of Mg2+ that is 2 N with respect to a Cl- ion, is only 1 N with respect to an O2- ion. Since 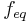 may not be unequivocal, IUPAC and NIST discourage the use of normality. [1]
may not be unequivocal, IUPAC and NIST discourage the use of normality. [1]
References
|
||||||||||||||